Praseodymium is a soft, ductile, and malleable metal with a subtle silvery shine. When it meets air, a green oxide layer forms gently on its surface. This metal is part of the lanthanide family, where it plays its role among other rare-earth elements.
Praseodymium has an average abundance of about 9.1 parts per million in the Earth's crust. It is not as "rare" as the name "rare-earth" might imply, but its dispersed occurrence makes economical extraction challenging. You can find it naturally in minerals such as monazite and bastnaesite. Its appearance and behavior in nature have drawn the attention of chemists and engineers alike over many decades.
Back in the mid-1800s, a Swedish doctor and chemist named Carl Gustav Mosander noted the existence of what he called “Didymium.” At that time, its properties seemed quite like those of lanthanum, and many believed it was a mixture of several elements. It was not until the later work of an Austrian chemist, Carl Auer von Welsbach, that the truth became clear. Around 1885, by dissolving double ammonium nitrates in nitric acid and separating the resulting mixture carefully through fractional crystallization, he distinguished that Didymium was actually made up of two distinct elements. One part exhibited bright green oxides, and it was given the name praseodymium—a name reflecting its light green hue combined with its identity as a twin element. The other part was later known as neodymium. This careful work opened up new chapters in the study and use of rare-earth metals.
In many practical situations, praseodymium finds its role not by flashy appearances but through steady contributions. One well-known function is in turning glass into a light green color. The first instance of such color treatment was seen at a famous glassworks in what is now the Czech Republic. In simple terms, praseodymium adds a unique tint to glass, making it stand out. Along with modifying the color of glass, compounds of this metal help in tinting ceramics with a charming yellow shade.
Beyond coloration, praseodymium partners with its twin, neodymium, to create strong magnets and specialized alloys. These alloys are found in high-performance parts such as aircraft engine components. There is also an interesting role for praseodymium in the film industry. It is used in carbon arc lights that shine from film projectors and studio equipment, contributing to the vivid and dynamic lighting needed on set.
Praseodymium oxide (Pr₆O₁₁), particularly when combined with ceria (CeO₂), forms a highly effective oxidation catalyst. These catalysts are crucial in automotive catalytic converters for oxidizing residual carbon monoxide and in industrial processes for treating volatile organic compounds (VOCs). The gentle yet reliable nature of these catalysts helps drive chemical reactions under controlled conditions.
One of the most curious properties of praseodymium comes from its effect on light. When praseodymium ions are doped into certain crystal hosts (like yttrium silicate), they can exhibit unique optical properties. This makes them suitable for solid-state lasers and potential applications in quantum memory, where the propagation of light can be dramatically manipulated under specific conditions. For someone who has spent many years in the laboratory, this is a fascinating reminder of how small changes at the atomic level can lead to surprising macroscopic effects.
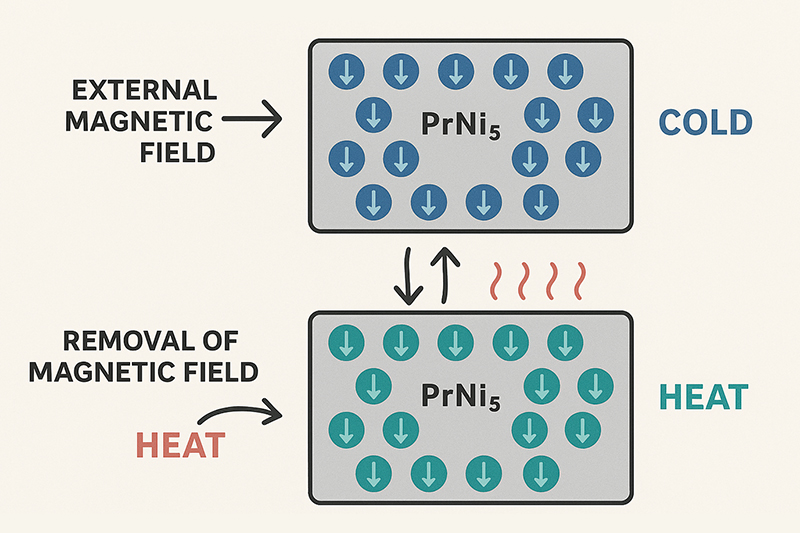
The intermetallic compound PrNi₅ is a classic example that exhibits a strong magnetocaloric effect at very low temperatures. It is a prime illustration of how praseodymium creates cold from magnetism: when the external magnetic field is removed during the adiabatic demagnetization process, the material absorbs heat from its surroundings, thereby generating intense cold. Ongoing research also explores praseodymium-based alloys for near-room-temperature magnetic refrigeration, which promises higher efficiency and eco-friendliness compared to conventional gas-compression systems.
Over many years of observation in both academic research and industrial practice, praseodymium serves in roles that might be easily overlooked at first glance. Its modest physical appearance hides a series of remarkable behaviors. When used in specialized magnets or custom alloys, this metal shows how nature’s gentle materials often provide the secret behind high technology.
In everyday settings, the coloring effects provided by praseodymium add a unique visual appeal to glass and ceramics. For instance, decorative objects and artistic installations sometimes feature praseodymium to evoke a warm, natural green glow that is both subtle and appealing. In a re-creation of historical lighting techniques, carbon arc lights enriched with praseodymium continue to provide robust and reliable performance even in the challenging conditions of a film studio.
The practical benefits of praseodymium are not limited to aesthetics. In magnetic cooling systems, for example, a small change in a magnetic field can lead to significant temperature variations. This magnetocaloric property serves as a cornerstone for research in low-temperature physics. In many cases, such properties open the door to innovative techniques for refrigeration in scientific instruments. It is a fine example of how basic research into a single element can translate into technology that pushes the boundaries of what is possible.
The extraction and purification of praseodymium have also improved over time. Modern advances in metallurgy ensure that this rare-earth metal is available in the high-purity forms needed by industries and research institutions. Even though praseodymium is not used in as many everyday products as some other metals, its specialized applications underscore its importance in select high-value fields.
In closing, praseodymium is a metal that quietly enriches our technological and artistic endeavors. More remarkably, its ability to generate cold from magnetism secures its indispensable role in fields ranging from low-temperature physics to quantum computing.
The exploration of elements like praseodymium is driven by both academic curiosity and industrial innovation. For researchers and engineers seeking high-purity praseodymium metals, alloys, and compounds to push the boundaries of their work, Stanford Materials Corporation (SMC) offers a reliable supply chain and technical expertise to support your endeavors.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.