
Lanthanum, a silvery-white rare earth metal, plays a crucial role in various modern technological advancements. Its unique properties and applications make it indispensable in numerous industries. This article delves into the properties of lanthanum and explores its diverse applications.
Lanthanum is characterized by its soft, malleable, and ductile nature. It has a silvery-white appearance, although it tarnishes rapidly when exposed to air, forming a thin oxide layer. The key physical properties of lanthanum include:
Lanthanum is the first element in the lanthanide series, and its electronic configuration is [Xe] 5d1 6s2. This configuration contributes to its chemical reactivity and the formation of various compounds and alloys.
Lanthanum exhibits several notable chemical properties, such as:
One of the primary applications of lanthanum is in the field of catalysis. Lanthanum-based catalysts are essential in refining petroleum. They enhance the catalytic cracking process, which breaks down complex hydrocarbons into simpler molecules, improving the efficiency of fuel production. Lanthanum oxide (La₂O₃) is a key component in these catalysts, known for its ability to increase the activity and stability of the catalytic material.
In the petroleum industry, lanthanum oxide is used in fluid catalytic cracking (FCC) units. FCC is a critical process in converting heavy crude oil fractions into valuable gasoline, diesel, and other lighter products. The addition of lanthanum to FCC catalysts improves their resistance to deactivation by sulfur and nitrogen compounds, thereby extending the catalyst's life and enhancing the overall efficiency of the process.
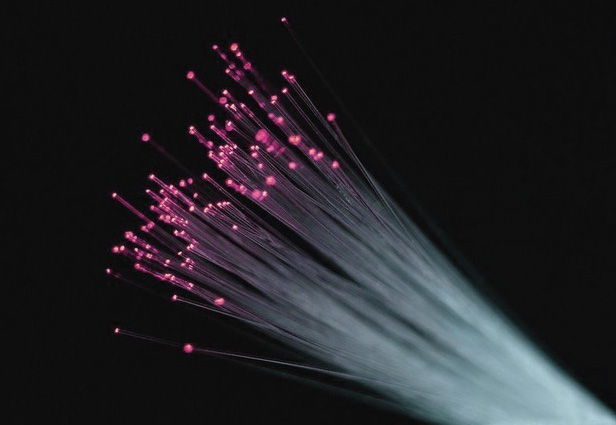
Lanthanum oxide is a key component in the production of specialized glass, including camera lenses and optical fibers. It improves the glass’s refractive index and reduces dispersion, leading to clearer and more precise optical instruments. High-refractive-index glass made with lanthanum oxide is used in advanced camera and microscope lenses, providing superior image quality and clarity.
In the telecommunications industry, lanthanum-doped optical fibers are used to improve signal transmission over long distances. The addition of lanthanum reduces signal loss and enhances the performance of fiber optic cables, making them essential for high-speed internet and data transmission networks.
Lanthanum plays a significant role in hydrogen storage technologies. Lanthanum-nickel alloys are used in metal hydride batteries, which store hydrogen efficiently. These batteries are vital for various applications, including electric vehicles and portable electronics. Lanthanum's ability to absorb and release hydrogen at relatively low pressures and temperatures makes it an ideal material for this purpose.
Nickel-metal hydride (NiMH) batteries, which contain lanthanum, are widely used in hybrid vehicles and portable electronic devices. These batteries offer higher energy density and longer lifespan compared to traditional nickel-cadmium (NiCd) batteries. The presence of lanthanum in the alloy improves the battery's overall performance and stability.
Lanthanum is integral to the electronics industry. It is used in the production of phosphors for color television and LED lights. Additionally, lanthanum’s magnetic properties make it valuable in manufacturing components for computers and other electronic devices.
Lanthanum oxide is used in the production of phosphors, which are materials that emit light when exposed to radiation. Phosphors containing lanthanum are used in color television screens, fluorescent lamps, and LED lights. These materials enhance the brightness and color quality of the displays, providing a better viewing experience.
Lanthanum is used in the production of magnetic materials, such as lanthanum-cobalt ferrites, which are essential components in high-performance magnets. These magnets are used in various electronic devices, including hard disk drives, electric motors, and sensors, due to their strong magnetic properties and stability.
In the medical field, lanthanum carbonate is used as a phosphate binder for patients with chronic kidney disease. It helps to control phosphate levels in the blood, reducing the risk of complications associated with high phosphate levels.
Lanthanum carbonate (La₂(CO₃)₃) is an effective phosphate binder used in the treatment of hyperphosphatemia, a condition characterized by elevated phosphate levels in the blood. Patients with chronic kidney disease often experience difficulty in maintaining proper phosphate balance, and lanthanum carbonate helps prevent the absorption of phosphate from the digestive tract, thereby lowering blood phosphate levels.
Lanthanum compounds are used in water treatment processes. Lanthanum chloride and lanthanum sulfate are effective in removing phosphate from water, preventing the growth of harmful algae in water bodies.
Lanthanum-based materials are used in environmental remediation to remove contaminants from water. Lanthanum chloride (LaCl₃) and lanthanum sulfate (La₂(SO₄)₃) are employed in the removal of phosphate from wastewater, preventing eutrophication, which is the excessive growth of algae due to high nutrient levels. These compounds bind with phosphate ions, forming insoluble lanthanum phosphate, which can be easily removed from the water.
Lanthanum is the first element in the lanthanide series, which includes 15 elements from lanthanum (La) to lutetium (Lu). These elements share similar properties but also have unique characteristics that make them suitable for various applications. Some notable lanthanides include:
Cerium is used in catalytic converters in automobiles to reduce harmful emissions. It is also a key component in the production of cerium oxide nanoparticles, which are used in fuel cells and to polish glass and semiconductors.
Neodymium is widely known for its use in powerful permanent magnets, which are essential in the production of wind turbines, electric vehicle motors, and various electronic devices.
Europium is a crucial element in the manufacturing of phosphors used in television screens and LED lights. It is responsible for producing the red and blue colors in these displays, contributing to their vibrant quality.
Gadolinium is used in medical imaging, particularly in magnetic resonance imaging (MRI) contrast agents. It enhances the quality of MRI scans by improving the contrast between different tissues in the body.
Terbium is used in solid-state devices and as a dopant in various materials to improve their luminescent properties. It is also used in green phosphors for color displays and fluorescent lamps.
These lanthanides, along with lanthanum, form a group of elements with unique properties that are crucial for various technological advancements. Their applications span across multiple industries, highlighting the importance of rare earth elements in modern science and technology.
Lanthanum, with its unique physical and chemical properties, has become a cornerstone in various industrial applications. From catalysis and optics to electronics and medicine, the versatility of lanthanum continues to drive innovation and technological advancements. By understanding the properties and applications of lanthanum and comparing it with other lanthanides, we can appreciate its significance in our daily lives and its potential to contribute to future technological breakthroughs.
Whether in the form of advanced catalysts, high-performance batteries, or cutting-edge medical treatments, lanthanum's impact on technology and society is profound and far-reaching. Companies like Stanford Materials Corporation (SMC) play a crucial role in supplying high-quality lanthanum, ensuring its benefits are fully realized across different applications. The continued exploration and utilization of lanthanum and its lanthanide counterparts promise to further enhance our capabilities and improve the quality of life in the years to come.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.