Rare earth elements refer to 15 lanthanides plus scandium and yttrium. They have good chemical and physical properties and thus are applied in various industries. One of its main uses is in the manufacturing of batteries. Adding rare earth materials to the battery can greatly increase the battery capacity and use times, reduce the weight of the battery, and not pollute the environment. This article will focus on rare earth elements used in VRLA batteries.
Valve-regulated lead-acid (VRLA) battery is a sealed valve-regulated lead-acid battery [1]. It is based on AGM (Absorbed Glass Mat) technology or colloidal GEL electrolyte.
The advantages of VRLA batteries are as follows:
1) Special structure, suitable for installation in any direction,
2) Superior high-current discharge characteristics,
3) Long service life, and
4) Its system can convert the generated gas into water, so there is no need to add water during use, and the operation and maintenance are relatively simple.
The wide application of this battery also benefits from the advantages mentioned above. VRLA batteries can be used in power tools, emergency lights, UPS, electric wheelchairs, computers and communication equipment, etc.
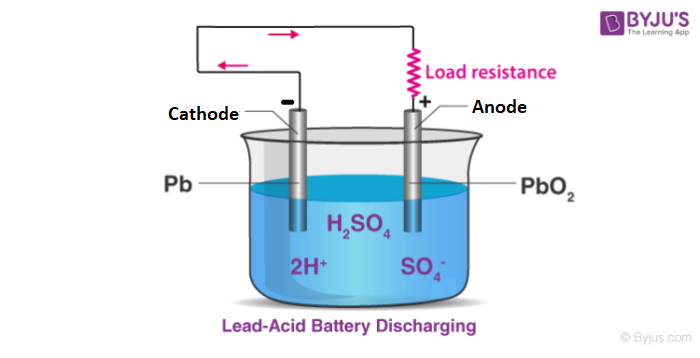
The basic electrode reaction of lead-acid batteries is the transformation between lead (Pb) and divalent lead (Pb) and tetravalent lead (Pb). Here are the equations of its discharge and charging process [2].
Discharge process:
(+) PbO2 + 4H+ + SO4- + 2e- <═══> PbSO4 + 2H2O (reduction)
(-) Pb + SO4 - - 2e-<═══> PbSO4 (oxidation )
The overall reaction equation is: PbO2 + 2H2SO4 + Pb <═══> 2PbSO4+ H2O
Charging process:
(+) PbSO4 + 2H2O - 2e- <═══> PbO2 + 4H+ + SO4- + 2e- (oxidation)
(-) PbSO4 + 2e- <═══> Pb + SO4- (reduction)
The overall reaction equation is: 2PbSO4+ H2O <═══> PbO2 + 2H2SO4 + Pb

The function of rare earth elements in VRLA batteries is mainly as grid material. The grid is the main component of the lead-acid battery. It is the current collector skeleton of the electrode, which conducts, collects, and distributes the current evenly. At the same time, it supports the active material and is the carrier of the active material. The positive electrode active material PbO2 in the lead-acid battery has poor conductivity and high resistivity, while the resistivity of the alloy grid is low, and the conductivity is more than 10,000 times that of PbO2. Alloy materials used as battery grids should have certain mechanical properties (such as hardness, tensile strength, elongation, etc.), corrosion resistance, electrical conductivity, excellent casting properties, and weldability.
At present, one of the grid materials of VRLA Battery is made of lead-calcium-tin alloy. The disadvantage of this material is that calcium is a relatively active metal, so it is easy to form a high-resistance anode corrosion layer on the surface of the grid, which hinders the deep cycle of the battery. Under this premise, rare earth alloy materials have been developed and used as grid materials in lead-acid batteries. Lead-rare earth alloy, as the positive grid material of VRLA, can effectively inhibit the corrosion of the anode, thereby increasing the cycle number and service life of the battery.
Yang et al. specifically studied the application of Pb-RE and Pb-Sn-RE alloy in VRLA batteries [3]. Among them, RE represents rare earth elements such as Lanthanum (La), Samarium (Sm), Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Gadolinium (Gd), Terbium (Tb), and Ytterbium (Yb). The experimental results show that some rare earth elements, such as Sm, Gd, and Tb, etc., have a significant inhibitory effect on the anodic corrosion of lead alloys in sulfuric acid solution. In other words, the addition of rare earth elements enhanced the corrosion resistance of lead alloys in batteries and suppressed the growth of high-resistance insulating layers.
In general, replacing the traditional Pb-Ca alloy with lead-rare earth alloy as the positive grid of VRLA Battery can significantly enhance the performance of the battery and prolong its service life. If you are interested in rare earth alloys or other REEs, please visit our website for more information. Stanford Materials Corporation (SMC) is a worldwide supplier of various rare earth oxides, metals, alloys, advanced ceramic materials, and minerals.
Reference:
[1] VRLA battery. (2022, December 18). In Wikipedia. https://en.wikipedia.org/wiki/VRLA_battery
[2] BYJU'S (n.d.). What is a Lead-acid Battery? https://byjus.com/chemistry/lead-acid-battery/
[3] Yang Chunxiao, Wang Huifeng, Zhou Yanbao, & Liu Houtian. (2005). Application of lead-rare earth alloys in VRLA batteries. Battery, 42(004), 147-149.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.

 Inquiry List
Inquiry List