
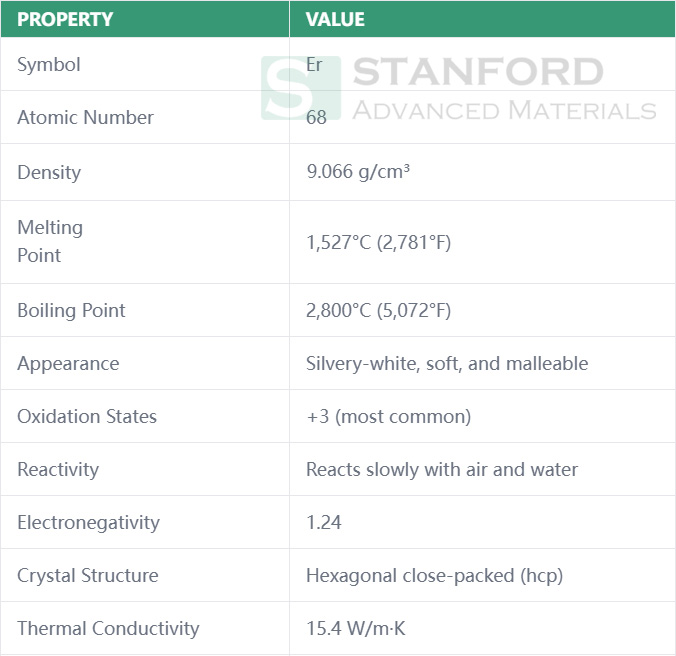
Erbium (Er) is a chemical element in the lanthanide series of the periodic table, classified as a rare-earth metal. With its distinctive silvery-white appearance, erbium is often overlooked in its metallic form due to its vulnerability to tarnish in air and corrosion in water. However, its unique chemical properties and compounds have found a range of important applications in industries such as optics, electronics, and metallurgy.
Discovered by Carl Gustaf Mosander in 1843, erbium plays a significant role in various technological fields, despite its limited use as a pure metal. In this article, we will explore the physical and chemical properties of erbium, its key compounds, and its diverse applications.
Appearance
Erbium is a soft, malleable, silvery-white metal. As a lanthanide, it shares many similarities with its rare-earth counterparts, including its soft texture and reactive properties.
Reactivity
Erbium is highly reactive, particularly in the presence of air and moisture. When exposed to oxygen, it forms a thin layer of oxide that causes it to tarnish. This tarnishing process, although slow, limits its widespread use as a pure metal in many applications. Additionally, erbium is attacked by water, which further hampers its utility in environments where corrosion resistance is a priority.
Alloying Properties
Erbium, when alloyed with other metals, has the ability to influence the physical properties of the resulting material. For example, when added to metals like vanadium, erbium can lower their hardness, making them more workable. This characteristic is valuable in the production of specialized alloys with improved machining characteristics, where a balance between strength and ease of shaping is essential.
Erbium Oxide (Er₂O₃)
One of the most important compounds of erbium is erbium oxide (Er₂O₃), which has a pale yellow or pinkish appearance. Erbium oxide is commonly used in the production of infrared-absorbing glass. These glasses are crucial in applications where protection from infrared radiation is necessary, such as in safety glasses for welders and metal workers. Erbium oxide’s ability to absorb infrared light makes it especially valuable in optical technologies.
Other Erbium Compounds
Erbium also forms a variety of other compounds, including erbium chloride (ErCl₃) and erbium fluoride (ErF₃). These compounds are used in a range of applications, particularly in the fields of lasers and electronics, where their unique optical and electronic properties can be harnessed.
Erbium is frequently doped into optical fibers for use in telecommunications and medical equipment. Erbium-doped fiber amplifiers (EDFA) are particularly important in boosting signal strength in long-distance fiber-optic communications.
Optical Applications
Erbium’s most significant application is in the field of optics, especially in the development of fiber optic communication systems. Erbium-doped fiber amplifiers (EDFAs) are widely used in telecommunications to boost signal strength in optical fiber networks. These devices rely on erbium’s ability to efficiently amplify light signals, allowing for faster and more reliable data transmission over long distances.
Laser Technology
Erbium is also a key component in laser technology, especially in the creation of solid-state lasers. Erbium-doped laser materials are commonly used in medical and dental applications, such as in laser surgery and teeth whitening, due to their efficiency in producing highly focused light. Erbium lasers are also used in spectroscopy and analytical instruments, where their precision and wavelength control are essential.
Metallurgy
In metallurgy, erbium is employed in alloying applications. When added to metals like vanadium or titanium, erbium improves their workability by reducing hardness and enhancing their ability to be shaped and processed. This makes erbium a valuable addition in the manufacturing of certain high-performance alloys used in aerospace and other specialized industries.
Infrared-Absorbing Glasses
Erbium oxide is utilized in producing infrared-absorbing glasses. These glasses are essential in safety applications, such as protective eyewear for welders and metal workers, where exposure to intense infrared radiation can be harmful. Erbium’s ability to selectively absorb infrared light makes it ideal for these protective applications.
Nuclear Applications
Although less common, erbium is also explored for its potential use in nuclear applications. Erbium’s ability to absorb neutrons has led to its study in reactor control and as a potential material in neutron-capture therapy for medical applications.
While erbium is not widely used in its pure metallic form due to its reactivity and tendency to tarnish, its compounds and alloys have found diverse and important applications across various industries. From fiber optics and laser technology to metallurgy and infrared protection, erbium continues to be an essential material in modern technology. Its unique properties, especially in the realm of optics and electronics, ensure that erbium remains a vital element in the development of future technological advancements.
Stanford Materials Corporation (SMC) plays a critical role in the advancement and distribution of rare earth materials, facilitating access to erbium for research and specialized applications. As scientific understanding deepens, responsible utilization and innovation may unlock even greater possibilities for this elusive element.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.

 Inquiry List
Inquiry List


