
Lanthanum chloride, a catalyst of considerable importance, plays a crucial role in diverse chemical reactions. This article delves into its mechanisms and wide-ranging applications in catalysis across industries. By understanding how lanthanum chloride functions as a catalyst, we can appreciate its pivotal role in driving forward industrial processes and scientific advancements.
From petrochemical refining to organic synthesis and environmental remediation, lanthanum chloride's versatile properties make it indispensable in facilitating key reactions that underpin various sectors. Lanthanum chloride plays a crucial role in catalysis. It contributes to the advancement of technology and innovation through its mechanisms and practical applications.
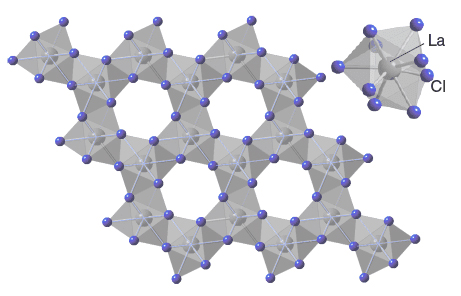
Lanthanum chloride, with the chemical formula LaCl₃, possesses distinctive chemical properties pivotal for its catalytic applications. Structurally, it comprises lanthanum ions (La³⁺) surrounded by chloride ions (Cl⁻), typically arranged in a hexagonal lattice structure, showcasing the adaptability and versatility of lanthanum ions in various chemical environments.
The chemical foundation of lanthanum chloride is the lanthanum ion, a trivalent cation from the lanthanide series. It exhibits a significant ionic radius, contributing to strong magnetic moments, particularly relevant in catalytic processes and applications like magnetic resonance imaging (MRI).
Reactivity and coordination chemistry are key aspects of lanthanum chloride's chemical behavior. It readily forms complexes with different ligands, allowing tailored designs in catalysis. Its Lewis acidic nature facilitates substrate activation in organic synthesis reactions, promoting new chemical bond formation.
Lanthanum chloride's coordination chemistry allows it to adopt various coordination numbers, typically ranging from 6 to 9. This flexibility enables the formation of complexes with diverse geometries and magnetic properties, optimizing its effectiveness in catalytic processes.
Lanthanum chloride serves as a catalyst through various mechanisms, each playing a crucial role in facilitating chemical reactions. One prominent mechanism involves Lewis acid catalysis, where it acts as an electron pair acceptor, promoting the formation of new chemical bonds. In this capacity, lanthanum chloride activates substrates by coordinating with electron-rich species, facilitating reactions such as Friedel-Crafts alkylation and acylation, Diels-Alder cycloaddition, and aldol condensation.
Additionally, lanthanum chloride participates in Lewis base catalysis, acting as an electron pair donor. In this role, it facilitates reactions by donating electron pairs to electron-deficient species, promoting transformations such as nucleophilic addition to carbonyl compounds and Michael addition reactions.
The Lewis acid-base duality of lanthanum chloride allows it to catalyze a wide range of chemical transformations, exploiting its versatile coordination chemistry and reactivity. By understanding these catalytic mechanisms, researchers can design and optimize reactions tailored to specific synthetic goals, harnessing the unique properties of lanthanum chloride to drive forward advancements in organic synthesis and industrial processes.
Lanthanum chloride (LaCl₃) serves as a versatile catalyst in various chemical reactions, owing to its unique chemical properties and reactivity. Its applications in catalysis span diverse fields, including petrochemical refining, organic synthesis, and environmental remediation, highlighting its significant contributions to industrial processes and scientific advancements.
Lanthanum chloride plays a crucial role as a catalyst in petrochemical refining processes, such as Fluid Catalytic Cracking (FCC), alkylation, isomerization, and polymerization reactions. In FCC, it enhances the conversion of heavy crude oil fractions into lighter, more valuable hydrocarbons like gasoline and diesel.
Additionally, it facilitates the production of high-octane alkylate gasoline in alkylation processes and promotes the formation of branched-chain isomers with higher octane numbers in isomerization reactions. Moreover, lanthanum chloride catalyzes the polymerization of olefins, contributing to the production of polymers and plastics with tailored properties.
In organic synthesis, lanthanum chloride serves as an effective catalyst in various reactions, including Friedel-Crafts alkylation and acylation, Diels-Alder reactions, aldol reactions, and the synthesis of heterocycles. It activates carbonyl compounds and facilitates the formation of carbon-carbon and carbon-oxygen bonds, enabling the efficient synthesis of complex organic molecules. Lanthanum chloride's Lewis acidity and versatile coordination chemistry make it particularly valuable in promoting selective reaction pathways and enhancing overall synthetic efficiency.
Lanthanum chloride contributes to environmental remediation efforts through catalytic processes aimed at pollution control. It aids in the removal of pollutants from water and air by catalyzing the conversion of harmful emissions, such as nitrogen oxides (NOx), volatile organic compounds (VOCs), sulfur dioxide (SO2), and phosphate ions. By promoting the breakdown of pollutants into less harmful substances, lanthanum chloride helps mitigate environmental pollution and protect natural ecosystems.
Lanthanum chloride has emerged as a versatile and potent catalyst, offering significant advantages across various chemical processes, from organic synthesis and petrochemical refining to environmental remediation. Its unique chemical properties, including high Lewis acidity and the ability to form stable complexes, have been leveraged to enhance reaction efficiencies, selectivities, and sustainability.
Despite challenges related to cost, availability, and environmental impact, the future of lanthanum chloride in catalysis is promising, with ongoing research aimed at overcoming these obstacles. Its continued development and application hold the potential to revolutionize catalytic processes, contributing to advancements in chemical manufacturing, pollution control, and beyond, underscoring its significant role in the future of catalysis.
Click here to inquire about high-quality lanthanum chloride powder and other rare earth compounds.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.

 Inquiry List
Inquiry List

