Gadolinium oxide (Gd2O3) occupies a unique position in the field of materials science, bridging the gap between fundamental research and cutting-edge applications. This rare earth oxide, derived from the element gadolinium, is renowned not just for its chemical stability and optical properties but also for its intriguing magnetic behavior.
Unlike its elemental form, which displays pronounced ferromagnetic properties under certain conditions, gadolinium oxide exhibits paramagnetism, a form of magnetism that is less understood and more subtle in its manifestation.
Read more: Ferromagnetic Vs. Paramagnetic Vs. Diamagnetic
The significance of Gd2O3 extends beyond mere academic curiosity; it plays a pivotal role in various technological applications, especially in enhancing the capabilities of magnetic resonance imaging (MRI) as a contrast agent.
This article aims to provide a comprehensive analysis of the magnetic properties of gadolinium oxide, exploring the underlying principles that govern its behavior and the implications for both science and technology. Through this detailed examination, we seek to illuminate the complex interplay between atomic structure and magnetic phenomena in this fascinating material.
Magnetism, a fundamental aspect of materials science, originates from the atomic level, primarily due to the behavior of electrons within atoms. Each electron carries a magnetic moment, a vector quantity that represents the electron's intrinsic magnetic field. These moments arise from two key sources: the electron's orbit around the nucleus and its inherent spin, a quantum mechanical property without classical analog. The collective interactions of these microscopic magnetic moments determine the macroscopic magnetic properties of the material.
Materials can be classified into several categories based on their magnetic behavior:
Quantum mechanics plays a pivotal role in explaining these phenomena, particularly through the principles governing electron spin and the Pauli exclusion principle, which dictates that no two electrons can occupy the same quantum state simultaneously. Understanding the quantum mechanical underpinnings of magnetism allows scientists to predict and manipulate the magnetic behavior of materials, paving the way for advancements in storage media, sensing devices, and medical technology.
Gadolinium, a member of the lanthanide series, stands out for its exceptional magnetic properties. Unique among its peers, gadolinium exhibits ferromagnetic behavior below a certain temperature, known as the Curie temperature, which is around 20°C (68°F). This ferromagnetism is attributed to the alignment of magnetic moments within the material, a phenomenon that occurs due to the presence of unpaired electrons in gadolinium's 4f orbitals.
Ferromagnetism in gadolinium is particularly fascinating because it is rare for elements in the lanthanide series to exhibit this type of magnetic order. The ferromagnetic nature of gadolinium is a result of its electronic structure, which allows for a significant number of unpaired electrons.
These electrons generate magnetic moments that, under the right conditions, align parallel to each other, creating a strong internal magnetic field. This alignment leads to the material's magnetization, making gadolinium an important material in various applications, including in the enhancement of magnetic resonance imaging (MRI) technology and the manufacturing of magnetic refrigeration systems.
Understanding the ferromagnetic properties of gadolinium provides a foundation for exploring how these properties change when gadolinium is combined with oxygen to form gadolinium oxide. The transition from the metallic state to the oxide form significantly alters the magnetic behavior of the material, demonstrating the intricate relationship between chemical composition and magnetic properties. This transition is a key focus in the study of magnetic materials, as it highlights the complex interplay between atomic structure and magnetic phenomena.
The transition from elemental gadolinium to gadolinium oxide (Gd2O3) marks a significant shift in magnetic properties, a change driven by the process of oxidation. In its elemental form, gadolinium exhibits ferromagnetic properties due to the alignment of magnetic moments from unpaired electrons. However, when gadolinium is oxidized to form Gd2O3, the introduction of oxygen atoms alters the electronic structure and, consequently, the magnetic interactions within the material.
This alteration leads to gadolinium oxide displaying paramagnetic behavior, characterized by the material's magnetic moments aligning with an external magnetic field but only in the presence of that field. Unlike ferromagnetism, where magnetic properties persist even after the removal of the external field, paramagnetism in gadolinium oxide is a temporary state that dissipates without the sustaining influence of an external magnetic force. This shift highlights the profound impact of chemical changes on the magnetic properties of materials, illustrating how oxidation can transform a ferromagnetic metal into a paramagnetic oxide.
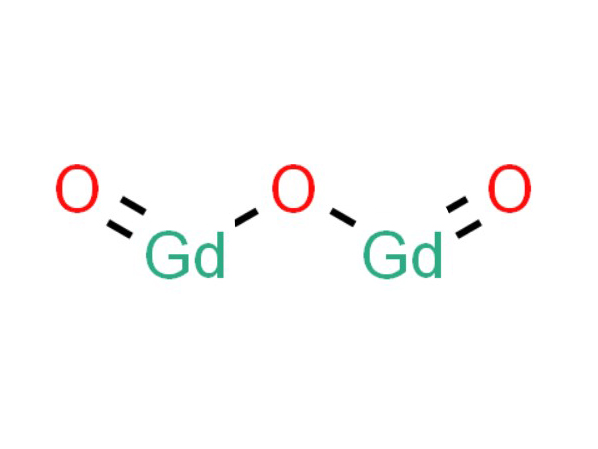
Gadolinium oxide's paramagnetic properties emerge from a distinct mechanism, diverging from the ferromagnetic characteristics of elemental gadolinium. This divergence is rooted in the quantum mechanical behavior of electrons within the gadolinium ions that comprise the oxide. Paramagnetism in Gd2O3 is primarily due to the presence of unpaired electrons in the gadolinium ions, which are capable of aligning their magnetic moments in response to an external magnetic field.
Quantum mechanics provides a framework to understand this alignment. According to quantum theory, each unpaired electron possesses a magnetic moment due to its spin, a fundamental property with no classical counterpart. In the absence of an external magnetic field, the spins of the unpaired electrons are randomly oriented, resulting in no net magnetic moment for the material.
However, when an external magnetic field is applied, these spins tend to align with the field, leading to a net magnetic moment in the direction of the field. This alignment is temporary and vanishes when the external field is removed, characteristic of paramagnetic behavior.
The contrast between gadolinium's ferromagnetism and gadolinium oxide's paramagnetism can be attributed to the different electronic structures facilitated by the oxidation process. In elemental gadolinium, the density of unpaired electrons and their interaction within the metal lattice promotes a spontaneous alignment of magnetic moments, leading to ferromagnetism. On the other hand, in gadolinium oxide, the spatial arrangement and electronic environment around the gadolinium ions limit such interactions, allowing only for temporary alignment under external magnetic influences.
This nuanced understanding of paramagnetism in gadolinium oxide not only highlights the intricate quantum mechanical interactions that govern magnetic behaviors but also underscores the significant impact of chemical composition on a material's magnetic properties. Through this exploration, the fascinating complexity of magnetic phenomena in the transition from elemental gadolinium to its oxide form is revealed, offering insights into the potential applications and theoretical implications of such materials.
The paramagnetic properties of gadolinium oxide have found critical applications in the field of medical imaging, particularly in magnetic resonance imaging (MRI). Gadolinium oxide's ability to enhance contrast in MRI scans stems from its paramagnetic nature, which interacts with the magnetic fields used in MRI to improve the visibility of internal structures. This enhancement is crucial for the accurate diagnosis of various conditions, aiding in the visualization of soft tissues that might otherwise remain indistinct.
Beyond medical imaging, gadolinium oxide's paramagnetic properties open doors to other innovative applications. In scientific research, gadolinium oxide can serve as a contrast agent in magnetic resonance microscopy, offering detailed insights at microscopic levels.
Additionally, its paramagnetic characteristics are being explored for potential use in advanced cooling technologies, such as magnetic refrigeration, where materials undergo temperature changes under varying magnetic fields. These applications not only highlight the practical benefits of understanding and harnessing the magnetic properties of gadolinium oxide but also underscore the material's versatility and the broad impact of paramagnetism in technology and healthcare.
In summary, this article has explored the intriguing magnetic properties of gadolinium oxide, from its fundamental paramagnetic behavior to its practical applications in fields as critical as medical imaging and beyond. Understanding the shift from ferromagnetism in elemental gadolinium to paramagnetism in its oxide form has revealed the significant impact of chemical composition on magnetic behaviors.
This comprehension not only enhances our grasp of quantum mechanical principles but also opens up avenues for technological and medical advancements. The exploration of gadolinium oxide's magnetic properties underscores the broader importance of material science in pushing the boundaries of what is possible, promising innovations and improvements in both current and future applications.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.