The third generation of fuel cells includes the solid oxide fuel cell (also known as SOFC), an all-solid-state device that efficiently and sustainably transforms chemical energy stored in fuel and oxidant into electrical energy at medium and high temperatures.
Similar to the proton exchange membrane fuel cell, solid oxide fuel cells are typically thought to be fuel cells that will be widely employed in the future.
A solid oxide fuel cell (SOFC) is an electrochemical device that uses the inclination of oxygen and hydrogen to react to transform the chemical energy in fuels into electrical energy.
The solid oxide fuel cell runs on air and a gaseous fuel like natural gas or biogas. They are unique in that they produce heat and electricity with a high level of efficiency while emitting no harmful emissions.
When installed in homes and buildings, for instance, they could supply hot water distributors, heating systems, and power networks.
Solid Oxide Fuel Cells. Image by: ResearchGate.
Solid oxide fuel cells (SOFCs), which have extraordinarily high electrical efficiency and low operating costs, have been regarded by experts for decades as having the most potential of any fuel cell technology.
Up to this point, significant work has gone into developing the electrolyte, anode, cathode, and connector materials for solid oxide fuel cells.
Solid oxide fuel cells were developed as a result of Nernst's 1899 proposal to use zirconia (ZrO2) as an oxygen ion conductor (SOFC).
In the late 1930s, Swiss scientists Emil Baur and H. Preis conducted research on solid oxide electrolytes using zirconium, yttrium, cerium, lanthanum, and tungsten as experimental materials.
O. K. Davtyan of Russia combined soda glass, tungsten trioxide, and sodium carbonate in the 1940s to " boost the conductivity and mechanical strength." However, Davtyan's ideas also had short life ratings and undesirable chemical interactions.
In the late 1950s, solid oxide technology development began to accelerate at the Central Technical Institute in The Hague, the Consolidation Coal Company in Pennsylvania, and General Electric in Schenectady, New York. According to a 1959 discussion of fuel cells, solid electrolytes have problems with melting, short-circuiting, and relatively high internal electrical resistance.
However, not everyone abandoned solid oxide. The rising energy costs and developments in material science have rekindled interest in SOFCs. A recent report identified about 40 companies developing these fuel cells.
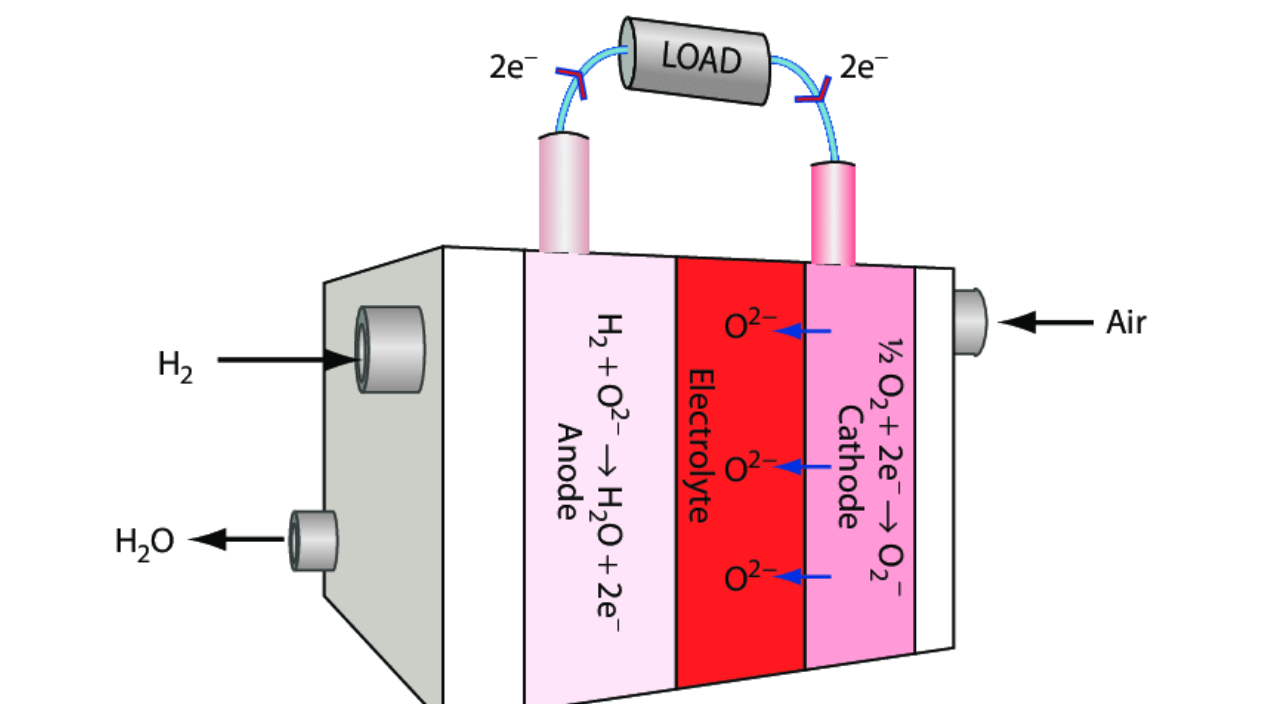
Three of the four layers that make up a solid oxide fuel cell are comprised of ceramic (hence the name). These four layers are piled together to form a single cell that is normally only a few millimeters thick.
Then, hundreds of these cells are linked together in series to create what is commonly known as a "SOFC stack." Because the ceramics used in SOFCs are not electrically and ionically active until very high temperatures, the stacks must operate at temperatures between 500 and 1,000 °C.
At the cathode, oxygen is reduced into oxygen ions. The fuel can then be electrochemically oxidized by these ions by diffusing through the solid oxide electrolyte to the anode. Two electrons and a byproduct of water are released in this process.
After that, these electrons move through an external circuit where they can act. Once more, those electrons enter the cathode material, and the cycle is completed.
They show higher efficiency in terms of both heat and power.
High efficiency has positive effects on the environment and the bottom line: the electrical efficiency of solid oxide fuel cells can approach 60%. This indicates that 60% of the fuel's stored energy gets transformed into usable electrical energy. Compared to coal power plants, this efficiency is substantially higher.
Solid oxide fuel cells typically use natural gas as a fuel as opposed to traditional energy sources that use batteries.
The total system can be expanded with additional fuel cell modules as necessary. This gives the user the financial freedom to match power generation investments to corporate needs.
Solid oxide fuel cells provide long-term stability, fuel flexibility, low emissions, and are relatively lower in cost.
In many different parts of the solid oxide fuel cells, rare earth elements are essential.
Scandium is a key element in SOFCs. It allows the electrolytes in the cells to run cooler, helping to reduce component costs and making these fuel cells competitive with other power sources.
Gadolinium-doped ceria has high ionic conductivity and is commonly used in oxygen sensors and solid oxide fuel cells.
Sodium terbium, when combined with zirconium dioxide, can act as a crystal stabilizer for high-temperature fuel cells.
Almost every component of solid oxide fuel cells now contains rare earth elements. The connections, anodes, solid electrolyte materials, and cathodes utilize rare earth metals. The roles of rare earth in SOFCs are as follows[1]:
1) Improve the ionic conductivity of the electrolyte materials by forming oxygen vacancies and defects.
2) Stabilize the materials structure of different components for the SOFCs.
3) Promote the mixed ionic-electronic conducting and the electrochemical performance of the electrodes including anode and cathode.
4) Improve the hydrothermal and chemical stability of the electrode materials under different conditions via formation of solid solutions with different phases.
5) Adjust the compatibility among all the components of the SOFCs.
Fuel cell technology makes it possible to generate electricity from fuels without combustion, more efficiently than with traditional generation systems, and with fewer emissions. But for them to work, suitable fuel is required.
Reference:
[1] Wang, Qi & Fan, Hui & Xiao, Yanfei & Zhang, Yihe. (2021). Applications and recent advances of rare earth in solid oxide fuel cells. Journal of Rare Earths. 40. 10.1016/j.jre.2021.09.003.
Eric Loewen
Eric Loewen graduated from the University of Illinois studying applied chemistry. His educational background gives him a broad base from which to approach many topics. He has been working with topics about advanced materials for over 5 years at Stanford Materials Corporation (SMC). His main purpose in writing these articles is to provide a free, yet quality resource for readers. He welcomes feedback on typos, errors, or differences in opinion that readers come across.

 Inquiry List
Inquiry List


